A meaningful survival benefit
HALAVEN® significantly extended life in third-line mBC that included all receptor subtypes1-4
UPDATED OS ANALYSIS (UNPLANNED): MEDIAN OS, MONTHS (95% CI)1,4,a
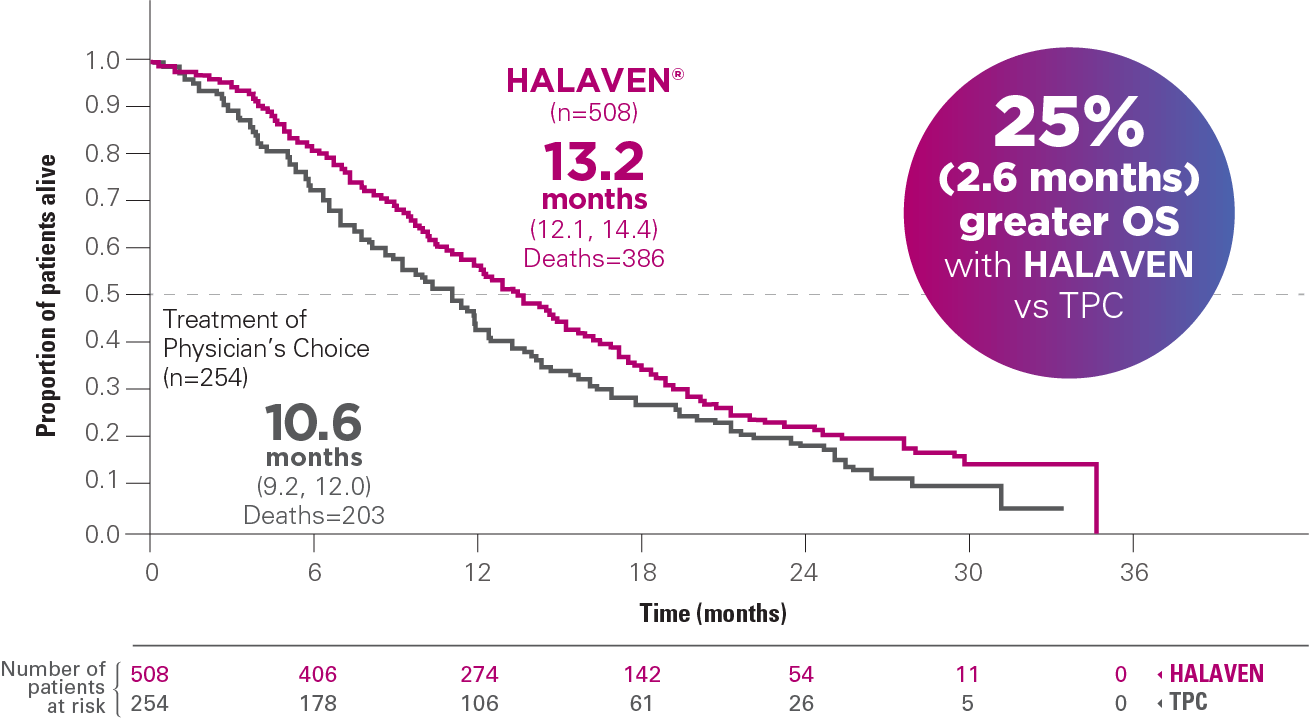
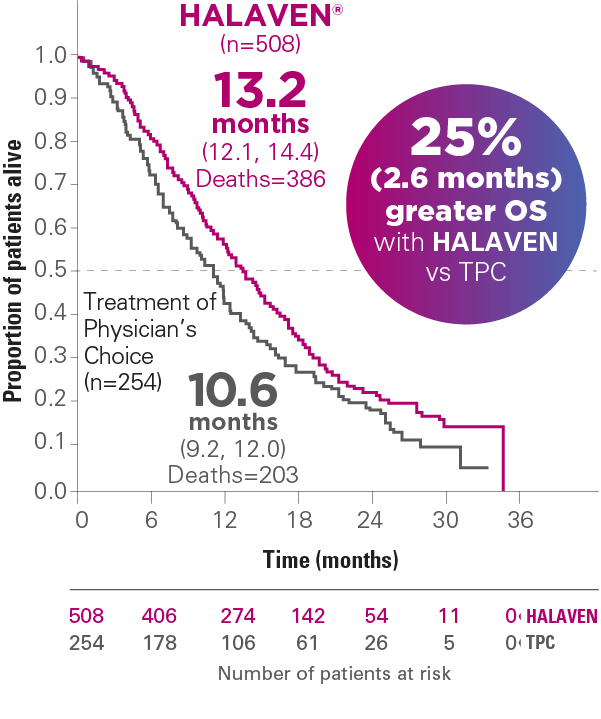
Results from an updated, unplanned survival analysis of the Phase III, randomized (2:1), open-label, multicenter, multinational EMBRACE trial of HALAVEN versus TPC (control arm) in patients with mBC (N=762), conducted when 77% of events (deaths) had been observed.1,4
mBC=metastatic breast cancer; OS=overall survival; CI=confidence interval; TPC=Treatment of Physician's Choice; EMBRACE=Eisai Metastatic Breast Cancer Study Assessing Physician's Choice E7389 (Eribulin).
aConducted in the intent-to-treat (ITT) population.
-
Results of the updated analysis were consistent with the primary analysis, which was conducted when ~50% of events (deaths) had been observed. HALAVEN demonstrated a median OS of 13.1 months (95% CI: 11.8, 14.3) vs 10.6 months with the TPC arm (95% CI: 9.3, 12.5), hazard ratio (HR)=0.81 (95% CI: 0.66, 0.99) (P=0.041)1,4
-
Reduction in risk of death—patients in the HALAVEN arm had a 19% reduction in risk of death vs real-world treatment options (defined as TPC)1,4-6
Indications
Metastatic Breast Cancer
HALAVEN (eribulin mesylate) Injection is indicated for the treatment of patients with metastatic breast cancer (mBC) who have previously received at least 2 chemotherapeutic regimens for the treatment of metastatic disease. Prior therapy should have included an anthracycline and a taxane in either the adjuvant or metastatic setting.
Liposarcoma
HALAVEN is indicated for the treatment of patients with unresectable or metastatic liposarcoma who have received a prior anthracycline-containing regimen.
Important Safety Information
Warnings and Precautions
Neutropenia: Severe neutropenia (ANC <500/mm3) lasting >1 week occurred in 12% of patients with mBC and liposarcoma or leiomyosarcoma. Febrile neutropenia occurred in 5% of patients with mBC and 2 patients (0.4%) died from complications. Febrile neutropenia occurred in 0.9% of patients with liposarcoma or leiomyosarcoma, and fatal neutropenic sepsis occurred in 0.9% of patients. Patients with mBC with elevated liver enzymes >3 × ULN and bilirubin >1.5 × ULN experienced a higher incidence of Grade 4 neutropenia and febrile neutropenia than patients with normal levels. Monitor complete blood cell counts prior to each dose, and increase the frequency of monitoring in patients who develop Grade 3 or 4 cytopenias. Delay administration and reduce subsequent doses in patients who experience febrile neutropenia or Grade 4 neutropenia lasting >7 days.
Peripheral Neuropathy: Grade 3 peripheral neuropathy occurred in 8% of patients with mBC (Grade 4=0.4%) and 22% developed a new or worsening neuropathy that had not recovered within a median follow-up duration of 269 days (range 25-662 days). Neuropathy lasting >1 year occurred in 5% of patients with mBC. Grade 3 peripheral neuropathy occurred in 3.1% of patients with liposarcoma and leiomyosarcoma receiving HALAVEN and neuropathy lasting more than 60 days occurred in 58% (38/65) of patients who had neuropathy at the last treatment visit. Patients should be monitored for signs of peripheral motor and sensory neuropathy. Withhold HALAVEN in patients who experience Grade 3 or 4 peripheral neuropathy until resolution to Grade 2 or less.
Embryo-Fetal Toxicity: HALAVEN can cause fetal harm when administered to a pregnant woman. Advise females of reproductive potential to use effective contraception during treatment with HALAVEN and for at least 2 weeks following the final dose. Advise males with female partners of reproductive potential to use effective contraception during treatment with HALAVEN and for 3.5 months following the final dose.
QT Prolongation: Monitor for prolonged QT intervals in patients with congestive heart failure, bradyarrhythmias, drugs known to prolong the QT interval, and electrolyte abnormalities. Correct hypokalemia or hypomagnesemia prior to initiating HALAVEN and monitor these electrolytes periodically during therapy. Avoid in patients with congenital long QT syndrome.
Adverse Reactions
In patients with mBC receiving HALAVEN, the most common adverse reactions (≥25%) were neutropenia (82%), anemia (58%), asthenia/fatigue (54%), alopecia (45%), peripheral neuropathy (35%), nausea (35%), and constipation (25%). Febrile neutropenia (4%) and neutropenia (2%) were the most common serious adverse reactions. The most common adverse reaction resulting in discontinuation was peripheral neuropathy (5%).
In patients with liposarcoma and leiomyosarcoma receiving HALAVEN, the most common adverse reactions (≥25%) reported in patients receiving HALAVEN were fatigue (62%), nausea (41%), alopecia (35%), constipation (32%), peripheral neuropathy (29%), abdominal pain (29%), and pyrexia (28%). The most common (≥5%) Grade 3-4 laboratory abnormalities reported in patients receiving HALAVEN were neutropenia (32%), hypokalemia (5.4%), and hypocalcemia (5%). Neutropenia (4.9%) and pyrexia (4.5%) were the most common serious adverse reactions. The most common adverse reactions resulting in discontinuation were fatigue and thrombocytopenia (0.9% each).
Use in Specific Populations
Lactation: Because of the potential for serious adverse reactions in breastfed infants from eribulin mesylate, advise women not to breastfeed during treatment with HALAVEN and for 2 weeks after the final dose.
Hepatic and Renal Impairment: A reduction in starting dose is recommended for patients with mild or moderate hepatic impairment and/or moderate or severe renal impairment.
Indications
Metastatic Breast Cancer
HALAVEN (eribulin mesylate) Injection is indicated for the treatment of patients with metastatic breast cancer (mBC) who have previously received at least 2 chemotherapeutic regimens for the treatment of metastatic disease. Prior therapy should have included an anthracycline and a taxane in either the adjuvant or metastatic setting.
Liposarcoma
HALAVEN is indicated for the treatment of patients with unresectable or metastatic liposarcoma who have received a prior anthracycline-containing regimen.
Indications
Metastatic Breast Cancer
HALAVEN (eribulin mesylate) Injection is indicated for the treatment of patients with metastatic breast cancer (mBC) who have previously received at least 2 chemotherapeutic regimens for the treatment of metastatic disease. Prior therapy should have included an anthracycline and a taxane in either the adjuvant or metastatic setting.
Liposarcoma
HALAVEN is indicated for the treatment of patients with unresectable or metastatic liposarcoma who have received a prior anthracycline-containing regimen.
Important Safety Information
Warnings and Precautions
Neutropenia: Severe neutropenia (ANC <500/mm3) lasting >1 week occurred in 12% of patients with mBC and liposarcoma or leiomyosarcoma. Febrile neutropenia occurred in 5% of patients with mBC and 2 patients (0.4%) died from complications. Febrile neutropenia occurred in 0.9% of patients with liposarcoma or leiomyosarcoma, and fatal neutropenic sepsis occurred in 0.9% of patients. Patients with mBC with elevated liver enzymes >3 × ULN and bilirubin >1.5 × ULN experienced a higher incidence of Grade 4 neutropenia and febrile neutropenia than patients with normal levels. Monitor complete blood cell counts prior to each dose, and increase the frequency of monitoring in patients who develop Grade 3 or 4 cytopenias. Delay administration and reduce subsequent doses in patients who experience febrile neutropenia or Grade 4 neutropenia lasting >7 days.
Peripheral Neuropathy: Grade 3 peripheral neuropathy occurred in 8% of patients with mBC (Grade 4=0.4%) and 22% developed a new or worsening neuropathy that had not recovered within a median follow-up duration of 269 days (range 25-662 days). Neuropathy lasting >1 year occurred in 5% of patients with mBC. Grade 3 peripheral neuropathy occurred in 3.1% of patients with liposarcoma and leiomyosarcoma receiving HALAVEN and neuropathy lasting more than 60 days occurred in 58% (38/65) of patients who had neuropathy at the last treatment visit. Patients should be monitored for signs of peripheral motor and sensory neuropathy. Withhold HALAVEN in patients who experience Grade 3 or 4 peripheral neuropathy until resolution to Grade 2 or less.
Embryo-Fetal Toxicity: HALAVEN can cause fetal harm when administered to a pregnant woman. Advise females of reproductive potential to use effective contraception during treatment with HALAVEN and for at least 2 weeks following the final dose. Advise males with female partners of reproductive potential to use effective contraception during treatment with HALAVEN and for 3.5 months following the final dose.
QT Prolongation: Monitor for prolonged QT intervals in patients with congestive heart failure, bradyarrhythmias, drugs known to prolong the QT interval, and electrolyte abnormalities. Correct hypokalemia or hypomagnesemia prior to initiating HALAVEN and monitor these electrolytes periodically during therapy. Avoid in patients with congenital long QT syndrome.
Adverse Reactions
In patients with mBC receiving HALAVEN, the most common adverse reactions (≥25%) were neutropenia (82%), anemia (58%), asthenia/fatigue (54%), alopecia (45%), peripheral neuropathy (35%), nausea (35%), and constipation (25%). Febrile neutropenia (4%) and neutropenia (2%) were the most common serious adverse reactions. The most common adverse reaction resulting in discontinuation was peripheral neuropathy (5%).
In patients with liposarcoma and leiomyosarcoma receiving HALAVEN, the most common adverse reactions (≥25%) reported in patients receiving HALAVEN were fatigue (62%), nausea (41%), alopecia (35%), constipation (32%), peripheral neuropathy (29%), abdominal pain (29%), and pyrexia (28%). The most common (≥5%) Grade 3-4 laboratory abnormalities reported in patients receiving HALAVEN were neutropenia (32%), hypokalemia (5.4%), and hypocalcemia (5%). Neutropenia (4.9%) and pyrexia (4.5%) were the most common serious adverse reactions. The most common adverse reactions resulting in discontinuation were fatigue and thrombocytopenia (0.9% each).
Use in Specific Populations
Lactation: Because of the potential for serious adverse reactions in breastfed infants from eribulin mesylate, advise women not to breastfeed during treatment with HALAVEN and for 2 weeks after the final dose.
Hepatic and Renal Impairment: A reduction in starting dose is recommended for patients with mild or moderate hepatic impairment and/or moderate or severe renal impairment.
References: 1. Cortes J, O'Shaughnessy J, Loesch D, et al; EMBRACE (Eisai Metastatic Breast Cancer Study Assessing Physician's Choice Versus E7389) investigators. Eribulin monotherapy versus treatment of physician's choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet. 2011;377(9769):914-923. 2. Saad ED, Katz A, Buyse M. Overall survival and post-progression survival in advanced breast cancer: a review of recent randomized clinical trials. J Clin Oncol. 2010;28(11):1958-1962. 3. Sparano JA, Vrdoljak E, Rixe O, et al. Randomized phase III trial of ixabepilone plus capecitabine versus capecitabine in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2010;28(20):3256-3263. 4. HALAVEN [package insert]. Nutley, NJ: Eisai Inc. 5. NCI dictionary of cancer terms. National Cancer Institute Web site. http://www.cancer.gov/publications/dictionaries/cancer-terms?CdrlD=618612. Accessed May 4, 2018. 6. Barraclough H, Simms L, Govindan R. Biostatistics primer: what a clinician ought to know: hazard ratios. J Thorac Oncol. 2011;6(6):978-982. 7. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines© ) for Breast Cancer V1.2018. © National Comprehensive Cancer Network, Inc. 2018. All rights reserved. Accessed April 19, 2018. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.

